Abstract
Background. The Sickle Cell Disease Implementation Consortium (SCDIC) was funded at eight sites in the United States by the National Heart Lung and Blood Institute (NHLBI) in 2016 to use implementation science to identify and address barriers to quality care in adolescents and adults with Sickle Cell Disease (SCD). As part of the SCDIC, the centers developed a SCD registry including 2444 persons (~300/site) ages 15-45 years at time of consent. To better understand the cause of death in adults living with SCD, this project assessed clinical co-morbidities among the enrolled adolescents and adults to identify causes of mortality and evaluate whether there are any identifiable clinical phenotypes at enrollment that would predict decreased survival.
Methods. At enrollment, data were collected, including patient reported demographics, and from the medical record: SCD phenotype, baseline laboratory values, comorbidities, and medication usage. Patient reported outcome (PRO) data were also collected regarding pain and quality of life. Mortality data were obtained for 84 adults who were enrolled since 2016 in the SCDIC registry. Subjects were followed for a median of 1.76 years. The relationship of clinical co-morbidities to mortality was determined using logistic regression after adjusting for SCD phenotype and age group. Specifically, a separate model was run for each co-morbidity, with SCD phenotype, age group, and the co-morbidity being retained in the model, regardless of significance. Summary statistics were calculated, including frequency and percentage for categorical variables and mean, standard deviation, median, and interquartile range (IQR) for continuous variables. Age was evaluated as a continuous and as a categorical variable (15-24, 25-34, 35-45 years).
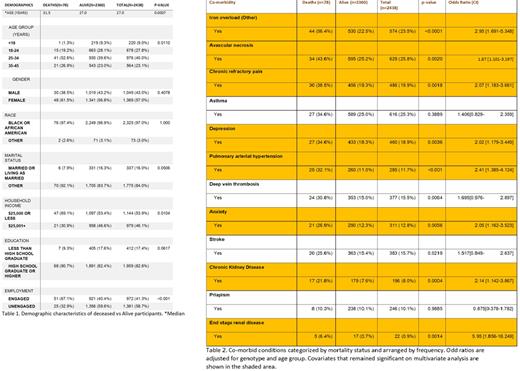
Results. A total of 2,444 registry participants were included for analysis. Eighty-four patients died over the course of the registry data collection (2017-2022), but six deaths were caused by trauma and were removed from further analysis. Baseline characteristics of the other 78 individuals who died (n=78, 3.2%) and those who are last known to be alive (n=2,360, 96.8%) are presented in Table 1. Several co-morbidities increase the odds of death, after adjusting for genotype and age group Table 2. These include, avascular necrosis in any joint (OR [95% CI], 1.87 [1.101-3.197]), chronic kidney disease (OR [95% CI], 2.14 [1.142-3.867]), end stage renal disease (OR [95% CI], 5.95 [1.856-16.249]), pulmonary arterial hypertension (OR [95% CI], 2.41 [1.385-4.124]), sarcoidosis (OR [95% CI], 18.61 [4.191-82.986]), iron overload (OR [95% CI], 2.95 [1.691-5.348]), chronic refractory pain (OR [95% CI], 2.07 [1.183-3.661]), anxiety (OR [95% CI], 2.05 [1.162-3.523]), depression (OR [95% CI], 2.02 [1.179-3.449]), and increased acute care utilization >10 hospitalizations in year prior to enrollment: 6.48 [2.400-16.998]; >10 visits to day-hospital/infusion center in year prior to enrollment: 5.11 [2.287-12.527]). Importantly, chronic pain, anxiety and depression were reported from the patient's past medical history and not abstracted from the PRO. Current use of hydroxyurea was not significant for the total study population (deaths vs survivors, p=0.0948). Among those who died, use of hydroxyurea was also non-significant for SCD phenotype [Hb SS vs Hb SC: p=0.0758 (current use), 1.000 (use within a year of death)].
Conclusion. SCD continues to reduce life expectancy for affected individuals. These findings support that clinicians and researchers still have much work to do in providing comprehensive and supportive care to prevent end-organ damage, comorbidities and SCD complications that are associated with decreased survival. These data also continue to demonstrate the need for improved mental health resources for this population.
Disclosures
Treadwell:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Glassberg:Novartis: Consultancy; CSL Behring: Consultancy. Kutlar:Eradigm Consultancy: Honoraria; American Society of Hematology: Consultancy, Honoraria, Research Funding; ORIC Pharmaceuticals, Inc.: Consultancy, Honoraria; PeerView Institute: Honoraria; MJH Life Sciences: Honoraria; Guidepoint Consulting Services: Consultancy, Honoraria; Novo Nordisk Pharmaceuticals: Honoraria, Research Funding; Forma Therapeutics, Inc.: Honoraria, Research Funding; Graphite Bio Inc.: Consultancy, Honoraria; bluebird bio Inc.: Consultancy, Honoraria; Vertex Pharmaceuticals, Inc.: Consultancy, Honoraria; Global Blood Therapeutics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Honoraria, Research Funding. Shah:CSL Behring: Consultancy; Bluebird Bio: Consultancy; Novartis: Research Funding, Speakers Bureau; Global Blood Therapeutics: Consultancy, Research Funding, Speakers Bureau; Alexion: Speakers Bureau. Gordeuk:Forma: Consultancy; GSK: Consultancy; GBT: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding. Hsu:Aruvant: Consultancy, Honoraria; DisperSol: Consultancy; Hilton Publishing: Consultancy, Research Funding; NIH: Research Funding; Sickle Cell Disease Association of America: Membership on an entity's Board of Directors or advisory committees; Sickle Cell Disease Association of Illinois: Membership on an entity's Board of Directors or advisory committees; Westat: Consultancy. Hankins:CVS Health: Consultancy; Forma Therapeutics: Consultancy; GBT: Consultancy; NHLBI: Membership on an entity's Board of Directors or advisory committees, Research Funding; CDC: Research Funding; ASH: Membership on an entity's Board of Directors or advisory committees; HRSA: Research Funding. King:Global Blood Therapeutics: Consultancy, Research Funding. Kanter:Graphite Bio: Consultancy; Beam: Honoraria; Guidepoint Global: Honoraria; Ecor1: Honoraria; GLG: Consultancy; Fulcrum Tx: Consultancy; Forma: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Alabama Birmingham: Current Employment; Novartis: Consultancy, Honoraria; ORIC: Consultancy; Bausch: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal